INFARMED Submissions & Regulatory Consulting
Navigating regulatory and reimbursement pathways is crucial for market access. We support clients in:
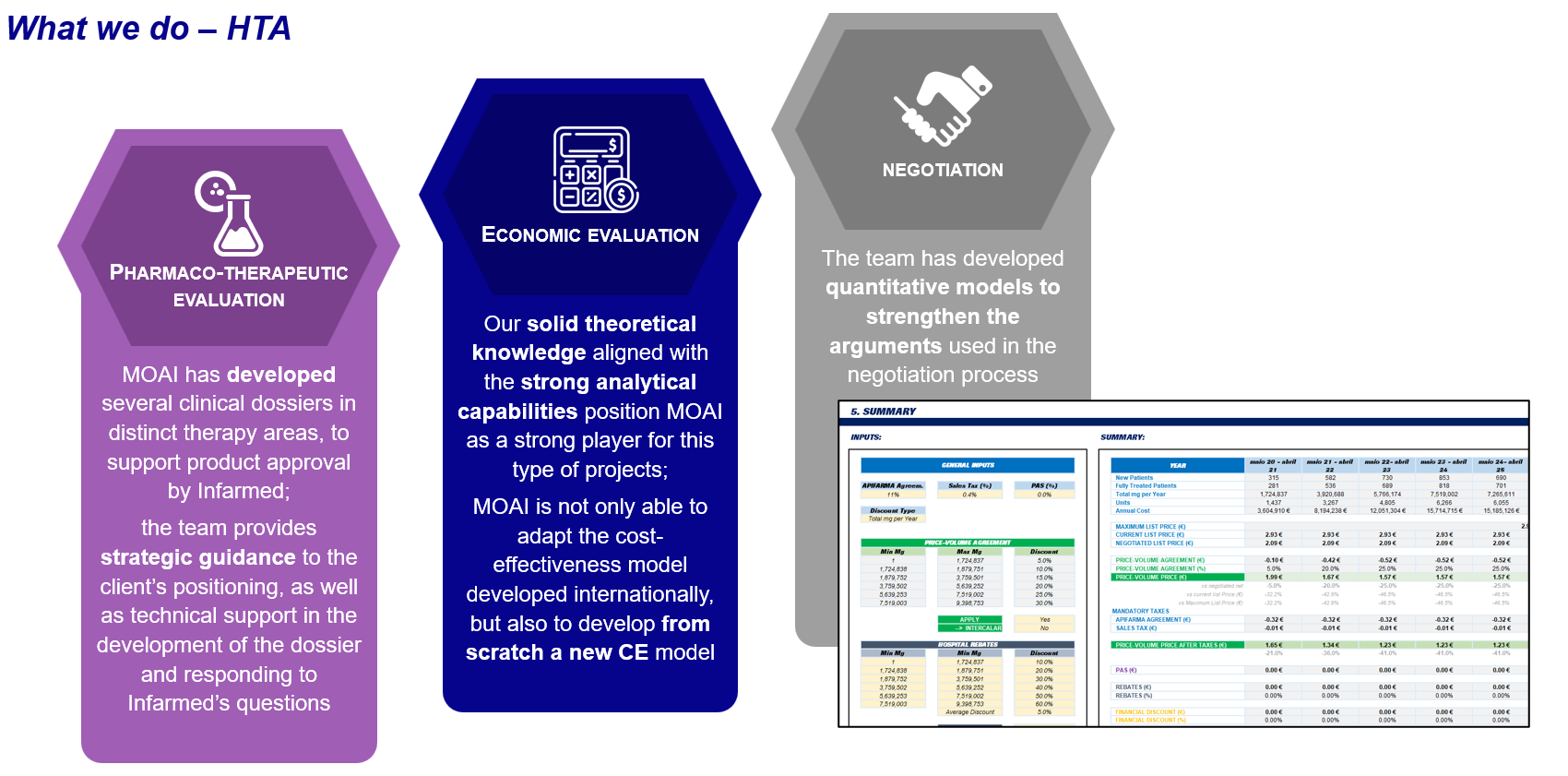
✔ Preparing and submitting pharmacotherapeutic evaluation dossiers for INFARMED approvals
✔ Conducting health technology assessments (HTA) to demonstrate cost-effectiveness and clinical value
✔ Supporting early dialogue with regulatory authorities to optimize market entry strategies
Our expertise ensures that new therapies and medical technologies reach the market efficiently, benefiting both patients and healthcare providers.